- Support portal
- Evaluation Kits and partner products
u-blox Support
- Product documentation
Documentation
- Investor relations
Investor relations
White paper
|
15 Sep 2016
Use Cases and Regulatory Approvals for Wireless Medical Devices
With the advent of wireless technology in medical applications, new possibilities open up for medical diagnostic and monitoring equipment in a hospital, health care facility, or for care in the home environment.
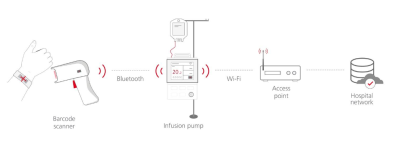
Medical device manufacturers need to understand the complex regulatory requirements, which cover several interrelated topics, such as radio/wireless compliance, medical device approval, hazard analysis, quality management and overall product safety.
Learn more and download our white paper here